
Top 20 Vaccines of 2024
Shots:
- Vaccines play an instrumental role in curbing infectious diseases by generating antibodies against antigens and creating a memory to defeat them. Several biopharma companies are steadfastly working to deliver vaccines against infectious and vaccine-preventable diseases around the world
- In 2024, the global vaccine market is estimated to be around $78B and is expected to reach $94.9B with a CAGR of 4% from 2024 to 2029. Comirnaty ranks first on our list with reported revenue of $11.22B, followed by Gardasil/Gardasil-9 and Spikevax
- PharmaShots brings a concise report on the top 20 vaccines based on total revenue generated in 2023
| S. No. | Products | Revenue (2022 $B) | Revenue (2023 $B) | Percent change (%) |
| 1 | Comirnaty | 37.80B | 11.22B | 70.31% |
| 2 | Gardasil/Gardasil-9 | 6.89B | 8.88B | 28.88% |
| 3 | Spikevax (mRNA-1273) | 18.4B | 6.7B | 63.58% |
| 4 | Prevnar Family | 6.33B | 6.44B | 1.73% |
| 5 | Shingrix | 3.57B | 4.38B | 22.68% |
| 6 | COVID-19 vaccine (Jcovden) | 2.17B | 1.11B | 48.84% |
| 7 | Bexsero | 0.91B | 1.08B | 18.62% |
| 8 | Varivax | 0.99B | 1.06B | 7.76% |
| 9 | Boostrix | 0.71B | 0.78B | 8.75% |
| 10 | Rotarix | 0.63B | 0.78B | 22.58% |
| 11 | RotaTeq | 0.78B | 0.76B | 1.78% |
| 12 | Infanrix/Pediarix | 0.71B | 0.7B | 1.87% |
| 13 | Fluarix/FluLaval | 0.86B | 0.64B | 25.73% |
| 14 | NVX-CoV2373 (Covovax, Nuvaxovid) | 1.55B | 0.53B | 65.82% |
| 15 | Menveo | 0.41B | 0.48B | 15.88% |
| 16 | Pneumovax 23 | 0.6B | 0.41B | 31.56% |
| 17 | Synflorix | 0.36B | 0.35B | 5.13% |
| 18 | Priorix | 0.22B | 0.33B | 48.31% |
| 19 | Ticovac | 0.2B | 0.26B | 34% |
| 20 | FluMist | 0.17B | 0.21B | 23.42% |
Note: Columns 1 and 2 represent ranks and products, while Columns 3, 4, and 5 showcase the total Oncology revenue of 2022, the total revenue of 2023, and the percentage change.

20. FluMist
Total Revenue: $216M
Company: AstraZeneca
First Approved: US (Jun 18, 2003), EU (Dec 4, 2013)
Indications Approved: Influenza
- FluMist, a quadrivalent live attenuated nasal spray flu vaccine, is used for active vaccination and prevention of influenza sickness caused by influenza A and influenza B subtype viruses
- FluMist protects against viral entrance and illness. The vaccination increases the synthesis of mucosal IgA, systemic IgG, and T lymphocytes
- In 2023, FluMist reported a revenue increase of 23.42% compared to 2022

19. TicoVac
Total Revenue: $268M
Company: Pfizer
First Approved: US (Aug 13, 2021), EU (N/A)
Indications Approved: Active immunization to prevent tick-borne encephalitis (TBE) disease
- TicoVac is an inactivated viral vaccine designed to prevent meningoencephalitis caused by the TBE) virus
- The vaccine comprises an inactivated entire virus, either the Neudoerfl or Karlsruhe (K23) strain of the TBEV-Eu subtype. It neutralizes antibodies to the natural TBE virus, protecting against tick-borne encephalitis (TBE)
- In 2023, TicoVac's revenue increased by 34% compared to 2022
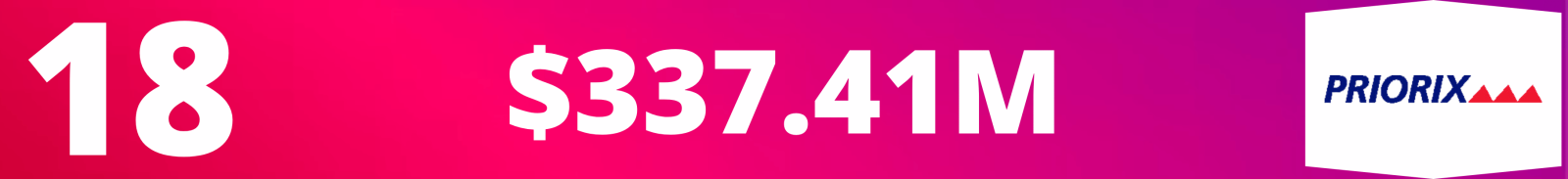
18. Priorix
Total Revenue: $337.41M
Company: GSK
First Approved: US (Jun 03, 2022), EU (May 25, 2012)
Indications Approved: Measles Prophylaxis, Mumps Prophylaxis, Rubella Prophylaxis
- Priorix is prescribed for active vaccination and prevention of measles, mumps, and rubella (MMR) in children aged 12 months and older
- Priorix is a lyophilized mixture of attenuated Schwarz measles, RIT 4385 mumps, and Wistar RA 27/3 rubella virus strains. The vaccination protects against MMR by neutralizing antibodies, which stimulate humoral immune responses against the MMR viruses
- In 2023, Priorix reported a 48.31% increase in revenue compared to 2022

17. Synflorix
Total Revenue: $350.14M
Company: GSK
First Approved: US (N/A), EU (Mar 30, 2009)
Indications Approved: Invasive Disease, Pneumonia, Acute Otitis Media
- Synflorix, a pneumococcal polysaccharide conjugate vaccine, is designed to treat invasive infections, pneumonia, and acute otitis media in infants and children aged six weeks to five years
- The vaccine contains ten active components, including S. pneumoniae polysaccharide serotypes 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, and 23F, each conjugated to a carrier protein (mostly protein D, tetanus toxoid, or diphtheria toxoid). The carrier protein helps T-cells and B-cells create a high-affinity antibody response to the polysaccharide antigen
- In 2023, Synflorix's revenue declined by 5.13% compared to 2022

16. Pneumovax 23
Total Revenue: $412M
Company: Merck & Co.
First Approved: US (Jun 01, 1983), EU (N/A)
Indications Approved: Provides immunization for the prevention of pneumococcal disease
- Pneumovax 23 is used against infections caused by the bacterium Streptococcus pneumoniae, including ear infections, sinus infections, pneumonia, blood infections, and meningitis
- Pneumovax 23 is an inactive vaccine that contains a mixture of 23 serotypes isolated from Streptococcus pneumoniae bacteria. After receiving the pneumococcal vaccination, the body detects these serotypes as foreign organisms and generates antibodies to combat them
- In 2023, Pneumovax 23's revenue decreased by 31.56% compared to 2022, owing to reduced demand in the U.S. as the market continues to transition toward newer adult pneumococcal conjugate vaccines

15. Menveo
Total Revenue: $483.83M
Company: GSK
First Approved: US (Feb 19, 2010), EU (Mar 15, 2010)
Indications Approved: Meningitis
- Menveo is used for active vaccination against invasive meningococcal illness caused by Neisseria meningitidis serogroups A, C, Y, and W-135 in people aged 2 months to 55 years
- The vaccine comprises tiny quantities of oligosaccharides from four serogroups (A, C, Y, and W-135) conjugated to a protein from the bacteria Corynebacterium diphtheriae. It works by preventing invasive meningococcal illness through the presence of serum bactericidal antibodies
- In 2023, Menveo's sales increased by 15.88% compared to 2022 due to the favorable impact of a US CDC (Center for Disease Control) stockpile replenishment

14. NVX-CoV2373 (Covovax, Nuvaxovid)
Total Revenue: $531.38M
Company: Novavax
First Approved: US (EUA Granted), EU (Jul 04, 2023)
Indications Approved: COVID-19
- NVX-CoV2373 received EUA (2023-2024 Formula) in the US for those 12 years of age and older. It is a protein-based vaccine that targets SARS-CoV-2, the virus that causes COVID-19
- Nanoparticles are created using recombinant technology, which is then combined with Matrix-M adjuvant to make the NVX-CoV2373 vaccine
- In 2023, NVX-CoV2373's revenue decreased by 65.82% compared to 2022 due to low demand for COVID-19 vaccines

13. Fluarix/FluLaval
Total Revenue: $641.71M
Company: GSK
First Approved: US (Dec 14, 2012), EU (Dec 12, 2018)
Indications Approved: Influenza
- Fluarix is approved for active vaccination in infants aged 6 months to adults aged 64 years against influenza illness caused by the influenza A and B virus subtypes
- Fluarix is an inactivated influenza vaccine comprising antigens grown in embryonated eggs and administered via intramuscular injection. The vaccination develops protection against the influenza virus by boosting the creation of disease-specific antibodies
- In 2023, Fluarix's sales declined by 25.73% compared to 2022, owing to competitive pressure and lower market demand in the US

12. Infanrix/Pediarix
Total Revenue: $705.38M
Company: GSK
First Approved: US (Jul 08, 2003), EU (Oct 23, 2000)
Indications Approved: Diphtheria, Tetanus, Pertussis, Polio, Hepatitis B, Haemophilus Influenza Type B
- Infanrix is approved for active immunization against diphtheria, tetanus, and pertussis in babies and children aged 6 weeks to 6 years, in a 5-dose course
- In contrast, Pediarix is recommended for active vaccination against diphtheria, tetanus, pertussis, and infections caused by all known subtypes of the hepatitis B virus. Both Infanrix and Pediarix provide protection by increasing antibody production and establishing immune memory
- In 2023, Infanrix and Pediarix's revenue declined by 1.87% compared to 2022

11. RotaTeq
Total Revenue: $769M
Company: Merck & Co.
First Approved: US (Feb 03, 2006), EU (Jun 27, 2006)
Indications Approved: Rotavirus Vaccine
- RotaTeq is a vaccination that prevents rotavirus gastroenteritis caused by strains G1, G2, G3, G4, and G9 in babies aged 6 to 32 weeks
- The vaccine contains a live, attenuated virus that replicates in the gut and interacts with the patient's immune system to induce immunity. The exact mechanism by which the rotavirus vaccination interacts with the immune system is not specified
- In 2023, RotaTeq's revenue declined by 1.78% compared to 2022

10. Rotarix
Total Revenue: $781.77M
Company: GSK
First Approved: US (Apr 03, 2008), EU (Feb 21, 2006)
Indications Approved: Rotavirus
- Rotarix inhibits rotavirus gastroenteritis caused by both G1 and non-G1 strains, such as G3, G4, and G9
- Rotarix is a live attenuated rotavirus vaccine developed from the human 89-12 strain of the G1P[8] type. Because the precise method by which Rotarix works is uncertain, it is assumed that the vaccine triggers neutralizing antibodies to boost immunity
- In 2023, the total revenue of Rotarix inclined by 22.58% as compared to 2022

09. Boostrix
Total Revenue: $781.77M
Company: GSK
First Approved: US (May 03, 2005), EU (Sep 24, 2010)
Indications Approved: Diphtheria, Tetanus, Acellular Pertussis Booster
- Boostrix is a non-infectious, sterile vaccine used to treat active booster vaccination against tetanus, diphtheria, and pertussis in people aged 10 and above
- It may also be used throughout the third trimester of pregnancy to prevent pertussis in infants under two months old. The vaccination works by inducing an antibody response to all the vaccine components
- In 2023, Boostrix revenue grew by 8.75% as compared to 2022

08. Varivax
Total Revenue: $1.06B
Company: Merck & Co.
First Approved: US (Mar 17, 1995), EU (Apr 21, 2021)
Indications Approved: Active Immunization of Varicella (Chickenpox)
- Varivax is used to help prevent varicella (chickenpox) in children 12 months of age and older
- The vaccine comprises a live, attenuated strain of the varicella-zoster virus (Oka/Merck strain). By presenting the body with a weakened form of the virus, Varivax stimulates the immune system to generate a protective response without causing the disease
- In 2023, Varivax's revenue increased by 4.94% compared to 2022

07. Bexsero
Total Revenue: $1.08B
Company: GSK
First Approved: US (Jan 23, 2015), EU (Jan 14, 2013)
Indications Approved: Meningitis
- Bexsero is a multicomponent meningococcal serogroup B vaccine designed for active protection against invasive diseases caused by Neisseria meningitidis serogroup B
- The vaccination stimulates the generation of antibodies against NHBA, NadA, fHbp, and PorA P1.4, proteins present on the surface of meningococci, and protects against invasive meningococcal illness, mostly by complement-mediated antibody-dependent death of N. meningitidis
- In 2023, Bexsero registered an increase of 18.62% in its revenue as compared to 2022 due to an increase, in sales because of its inclusion in National Immunization Programs in Europe

06. COVID-19 vaccine (Jcovden)
Total Revenue: $1.11B
Company: Johnson & Johnson
First Approved: US (Authorisation Revoked), EU (Jan 09, 2023)
Indications Approved: COVID-19
- Jcovden is recommended for the prevention of COVID-19 caused by SARS-CoV-2 in individuals aged 18 and older
- Jcovden is a monovalent vaccine made from a recombinant, replication-incompetent human adenovirus type 26 vector that encodes the SARS-CoV-2 full-length spike glycoprotein. Following delivery, the S glycoprotein of SARS-CoV-2 is transiently produced, producing immune responses against the S antigen as well as neutralizing and other functional S-specific antibodies that aid in the protection against COVID-19
- In 2023, Jcovden's revenue declined by 48.84% as compared to 2022 due to the lower demand for Covid-19 vaccines

05. Shingrix
Total Revenue: $4.38B
Company: GSK
First Approved: US (Mar 21, 2018), EU (Oct 20, 2017)
Indications Approved: Herpes Zoster (Shingles)
- Shingrix is a vaccine used against shingles (herpes zoster) and postherpetic neuralgia (PHN) that causes painful rashes, adults over 19 years can receive this vaccine
- It is a recombinant, non-live vaccine that stimulates the immune system to generate a strong and sustained immune response primarily evoking a specific immune response against varicella-zoster virus
- In 2023, Shingrix's revenue registered an incline of 22.68% vs. 2022 due to public funding expansion and strong private uptake globally

04. Prevnar Family
Total Revenue: $6.44B
Company: Pfizer, Daewoong
First Approved: US (Prevnar 13: Feb 24, 2010; Prevnar 20: Jun 08, 2021), EU (Prevnar 13: Feb 14, 2022; Prevnar 20: Dec 09, 2009)
Indications Approved: Pneumococcal Disease Prophylaxis
- The Prevnar Family comprises Prevnar 13 and Prevnar 20. Prevnar 13 is indicated for active immunization against invasive disease caused by Streptococcus pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A 19F, and 23F in individuals aged 6 weeks to 5 years, as well as the prevention of otitis media caused by serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F
- Prevnar 20 is approved for the prevention of invasive illness caused by serotypes 1, 3, 4, 5, 6A, 6B, 7F, 8, 9V, 10A, 11A, 12F, 14, 15B, 18C, 19A, 19F, 22F, 23F, and 33F in people 6 weeks of age and older. Prevnar 13 and Prevnar 20 operate by producing a T-cell-dependent immunological response, assisted by polysaccharides conjugated to a carrier protein
- In 2023, total revenue generated from the Prevnar Family increased by 1.73% as compared to 2022

03. Spikevax (mRNA-1273)
Total Revenue: $6.7B
Company: Moderna
First Approved: US (Jan 31, 2022), EU (Oct 03, 2022)
Indications Approved: COVID-19
- Spikevax is an mRNA vaccine that is delivered to people starting at the age of six months as two injections, generally into the muscle of the upper arm or the thigh in babies and young children, 28 days apart
- The mRNA provides the instructions for producing the spike protein, that is present on the surface of the SARS-CoV-2 virus. When a person receives the vaccination, some cells interpret the mRNA instructions and create the spike protein. The individual's immune system will then recognize this protein as alien, producing antibodies and activating T-cells to fight it
- In 2023, Spikevax's revenue dipped by 63.58% as compared to 2022, due to low demand for the COVID vaccine in 2023

02. Gardasil & Gardasil-9
Total Revenue: $8.88B
Company: Merck & Co.
First Approved: US (Gardasil-Jun 06, 2006; Gardasil-9- Dec 10, 2014), EU (Gardasil- Sep 20, 2006; Gardasil-9- Jun 10, 2015)
Indications Approved: Gardasil - Human Papillomavirus Vaccines and Gardasil-9 – Human Papillomavirus 9-Valent
- Gardasil is prescribed for human papillomavirus (HPV) in people aged 9 to 26. It is a non-infectious recombinant quadrivalent vaccination based on purified (VLPs) main capsid proteins from HPV types 6, 11, 16, and 18
- Gardasil-9 is used against HPV in people aged 9 to 45. It is derived from isolated VLPs of the main capsid proteins of HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58. Both vaccines work by stimulating a humoral immune response
- In 2023, total revenue of Gardasil and Gardasil-9 increased by 28.88% as compared to 2022, owing to the higher sales of both the vaccines

01. Comirnaty
Total Revenue: $11.22B
Company: Pfizer, BioNTech
First Approved: US (Aug 23, 2021), EU (Oct 10, 2022)
Indications Approved: COVID-19
- Comirnaty is a vaccine used for active immunization against COVID-19 caused by SARS-CoV-2 in people aged 12 and above
- The vaccine works by encapsulating the nucleoside-modified mRNA in lipid particles, allowing the mRNA to be delivered into host cells and expressed as the SARS-CoV-2 S antigen. It extracts both neutralizing antibodies and cellular immunological responses to the S protein, which aids in the protection against future SARS-CoV-2 infection
- In 2023, Comirnaty's sales decreased by 70.31% as compared to 2022 due to low demand of Covid vaccines
Sources:
- Annual reports
- SEC Filings
- Press releases
- Company websites
Market Cap Source: Google Finance (July 2024)
Currency Conversion: X-Rates (July 2024)
Related Posts: Top 20 Vaccines Based on 2022 Total Revenue
Tags

An avid reader and a dedicated learner, Prince works as a Content Writer at PharmaShots. Prince possesses an exceptional quality of breaking down the barriers of words by simplifying the terms in digestible chunks to make content readable and comprehensible. Prince likes to read books and loves to spend his free time learning and upskilling himself.














